Stability testing
& storage
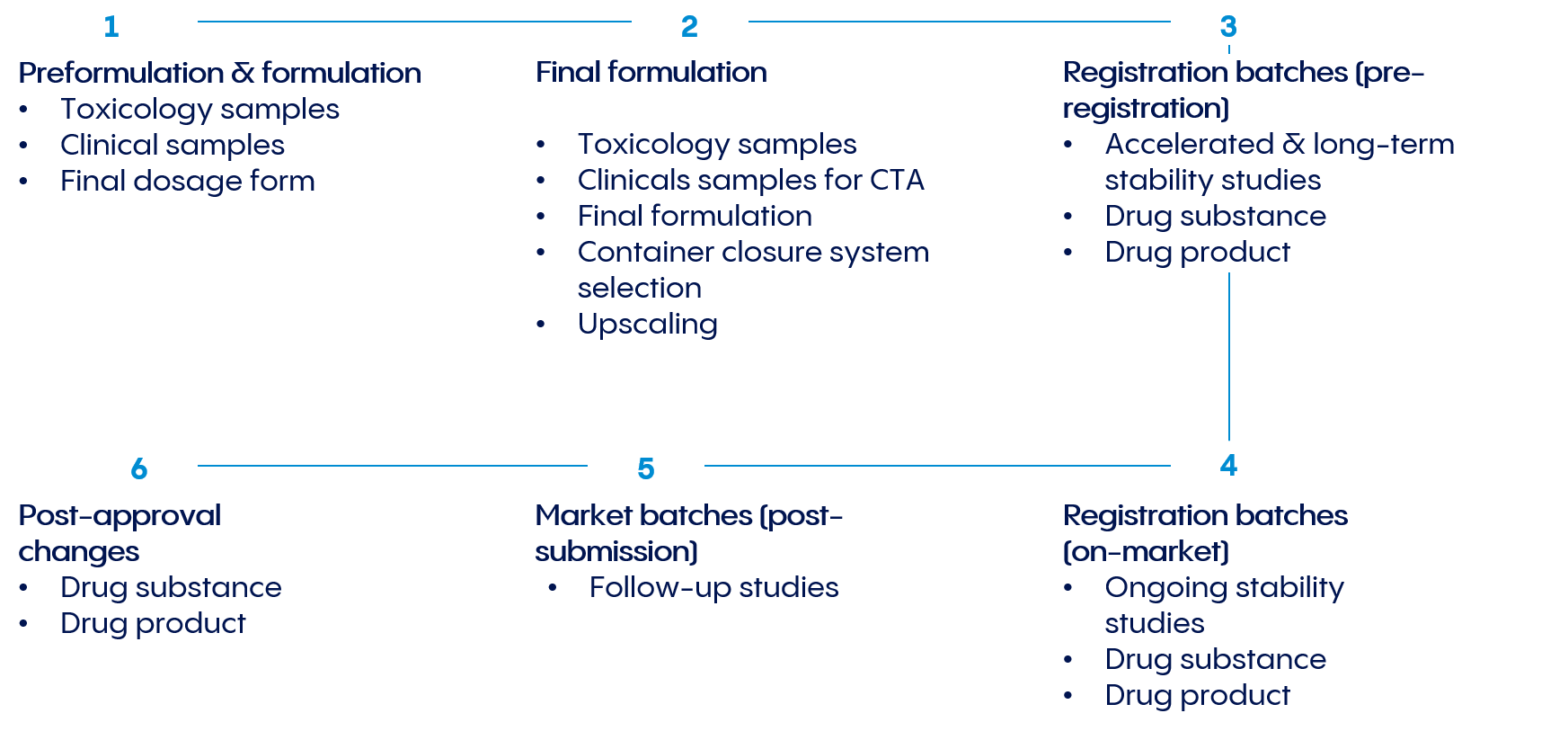
Throughout the product life cycle, we provide ICH-compliant stability programs, comprehensive stability testing, and sample storage that accommodates both standard temperature ranges and special product requirements.